Polycystic Ovary Syndrome
Polycystic ovary syndrome (PCOS) is an endocrine disorder with effects beyond fertility. Understanding the causes and how they can affect one’s health empowers us to create an innovative and effective treatment strategy allowing those with PCOS to live a full and fruitful life.
Dr. Awonuga is an expert in PCOS, and once he confirms the diagnosis, he always spends time dispelling some of the misinformation on the topic. Common misconceptions include:
1. PCOS is only a problem for overweight and obese women or that PCOS patients are obese: PCOS can affect people of all body sizes and many with PCOS are of average weight or even thin.
2. PCOS patients always have irregular menstrual periods: this is not true because there are four phenotypes (types) of PCOS, and one of these phenotypes has regular menstrual periods because they ovulate.
3. PCOS is caused by a specific type of diet or lifestyle: PCOS is a complex disorder that is thought to have many contributing genetic and epigenetic factors.
4. PCOS patients are infertile: many women with PCOS conceive naturally or with fertility treatments.
5. All women with PCOS have unwanted hair growth and acne: some may not experience these symptoms despite increased levels of male-type hormones in their blood.
6. Having PCOS means women have cysts in your ovaries: The so-called “cysts” are actually follicles. Follicles are fluid-filled sacs in the ovary that contain a single egg, and vary in size from less than 10 mm (the eggs within them are immature) when first recruited to 18 – 24 mm (contain a mature egg) at the time of ovulation. At the beginning of each menstrual cycle, women without PCOS recruit about 10 -20 follicles, while those with PCOS recruit more than or equal to 25 follicles. These follicles are best observed by performing a transvaginal pelvic ultrasound in the early phase of the menstrual cycle, usually between days 2 – 5 from the start of the period.

Ovary without PCOS with few follicles.
Ovary with PCOS with many follicles.
Diagnosing PCOS
The diagnostic criteria for PCOS have evolved over the years. What is known is that patients with PCOS can have:
1. Clinical evidence (hirsutism and acne) and or high blood levels of male hormones (biochemical high hyperandrogenemia).
2. Prolonged period of lack of ovulation (chronic anovulation) that presents as irregular or missed menstrual periods or a long time between periods (typically < 6 cycles per year).
3. On ultrasound, one or both ovaries have a polycystic-like appearance (PCO-like ovaries).
In 1990, the National Institutes of Health (NIH) first attempted to determine what constitutes PCOS, stating that only the first two criteria are needed to make the diagnosis. This was followed by an expert consensus conference sponsored by the European Society of Human Reproduction and Embryology (ESHRE) and the American Society for Reproductive Medicine (ASRM) in Rotterdam, Netherlands in 2003 that added the third criterion and stated that any two of the three criteria could be used to make the diagnosis because it was universally accepted that the Rotterdam criteria included milder phenotypes that increased the number of people diagnosed (prevalence) with PCOS, the Androgen Excess Society (AES) in 2006 recognized hyperandrogenism as a necessary diagnostic factor, which in combination with one of the two remaining criteria constitutes PCOS. This has led to four different phenotypes (types) of PCOS.
Types of PCOS
1. Phenotype A in which PCOS women have all three criteria, namely prolonged lack of ovulation (Chronic anovulation) and skin (hirsutism, acne, male-type baldness [alopecia], and velvety dark skin at the back of the neck and armpit), and or blood evidence of high male hormone (hyperandrogenemia), and PCO-like ovaries.
2. Phenotype B. Women have chronic anovulation and hyperandrogenemia only.
3. Phenotype C. Women have hyperandrogenemia and PCO-like ovaries but regular menstrual periods and thus ovulate.
4. Phenotype D. Women do not ovulate and have PCO-like ovaries but do not have hyperandrogenemia.
Given that PCO-like ovaries and irregular periods are not uncommon in teenagers, a diagnosis of PCOS shouldn't be made until late teenage years. For the diagnosis of PCOS, it is recommended that other conditions that can cause hyperandrogenism be ruled out. These conditions include:
1. Adult-onset congenital adrenal hyperplasia, characterized by high 17-hydroxyprogesterone due to 21-hydroxylase deficiency.
2. High prolactin (the hormone that is necessary for lactation) level, termed hyperprolactinemia
3. Thyroid disorders
4. Cushion Syndrome
5. Androgen-secreting tumors (neoplasms)
Evaluation of PCOS
Physical Examination
1. Measure Blood pressure 2. We calculate the woman’s body mass index (BMI, weight in kilograms divided by height in meters squared) 3. We measure her waist circumference to determine body fat distribution. A value greater than 35 inches is abnormal 4. We look for and record the presence of stigmata of hyperandrogenism and insulin resistance, such as acne, hirsutism, androgenic alopecia, and acanthosis nigricans
Laboratory Tests
1. Documentation of biochemical hyperandrogenism: we take blood to measure Total testosterone and sex hormone-binding globulin, or bioavailable and free testosterone 2. We take blood to exclude other causes of hyperandrogenism. These tests are best done in the morning and, in some cases, in the early part of the menstrual cycle. a. Thyroid dysfunction - thyroid-stimulating hormone (TSH) and free thyroxine (FreeT4) levels b. Hyperprolactinemia – prolactin c. Nonclassical congenital adrenal hyperplasia due to 21-hydroxylase deficiency – 17 hydroxyprogesterone (17-OHP) d. Cushing syndrome - random normal level or morning fasting level, followed by a twenty-four-hour urinary free-cortisol excretion test or a low–dose dexamethasone suppression if further testing is required.
Evaluate for metabolic abnormalities
1. Two-hour oral glucose tolerance test, fasting, and 2-hour insulin levels 2. Fasting lipid and lipoprotein level (total cholesterol, high-density lipoproteins, triglycerides, and low-density lipoproteins.
Ultrasound Examination performed in the early phase of the menstrual cycle (Days 2 – 5)
Initially, women are said to have polycystic ovaries when in one or both ovaries, there are 12 or more small, fluid-filled sacs (called antral follicles) measuring less than 10 mm in diameter, or increased ovarian volume (greater than 10 cm3). Because the follicle count of 12 led to overdiagnosis of PCOS, the threshold has been raised to ≥ 20.
Management of PCOS at MFS
Management depends on the woman's primary concern, and because Dr. Awonuga is a reproductive endocrinologist and infertility specialist, this may not necessarily include fertility. Thus, management is divided into two parts:
1. Ensuring women with PCOS are in good and optimal health and reducing long-term risk associated with PCOS
2. Management of subfertility in women with PCOS.
Examples of management efforts
At Michigan Fertility Services, we encourage and emphasize lifestyle changes e.g., healthy low carbohydrate, low fat, particularly saturated fat and high protein and high fiber diet, regular exercise, cessation of smoking and alcohol consumption. These do several things in women with PCOS. 1. Weight loss This would help the client achieve a healthy weight. Achieving a BMI as close to the ideal (19 to 25) is associated with better chances of conception and a healthier pregnancy. Weight loss increases serum hormone-binding globulin, which helps bind male hormones (androgens) in PCOS women. This helps reduce free androgen levels in PCOS and decreases androgen stimulation of hair and skin. Weight loss also improves ovulatory function in PCOS women, thereby increasing conception rates and possibly decreasing the risk of miscarriage. Occasionally, some PCOS women may need to have bariatric Surgery, especially when dietary measures have failed in those who are excessively obese. These patients should be aware that they will not be able to embark on pregnancy until 18 months after surgery. 2. Dietary changes Incorporating a diet to reduce saturated fat intake, increase protein, and increase fiber along with exercise can help lower elevated lipids, such as high total cholesterol, triglycerides, and low high-density lipoproteins, as seen in some women with PCOS. 3. Use of Metformin. Metformin, termed an ‘insulin sensitizer’, increases glucose receptor (a molecule in or on the surface of a cell that binds to a specific substance and causes a specific effect or action in the cell) sensitivity to insulin and will increase glucose absorption from the gastrointestinal tract and uptake by the liver. This allows for better overall blood glucose control, avoiding the hills and valleys of blood sugar levels. Because insulin also stimulates the ovaries to produce androgens, decreasing insulin levels by this medication will further decrease androgen production, making it easy for women to respond to ovulation induction medications. *Dr. Awonuga does not routinely prescribe Metformin to PCOS women. He does this only in those who have been proven to be insulin resistant based on the results of a 2-hour glucose tolerance test and insulin levels, not based on hemoglobin A1C (HbA1c is a blood test that determines average blood sugar [glucose] level over the previous 2 – 3 months) levels alone. Metformin, in combination with dieting and exercise, would be additive in controlling PCOS women’s glycemic dynamics. 4. Diabetes management Some women will already have type 2 diabetes when they show up at MFS. Type 2 diabetes, also termed non-insulin dependent diabetes mellitus (NIDDM), may be due to the pancreas not producing enough insulin or due to the amount of insulin being produced not being enough to counter the prevailing insulin resistance (IR) in PCOS and obese patients. Even in this group of women, the initial treatment is with diet and exercise. While metformin is the initial drug of choice in mild NIDDM resulting from IR, Glyburide would be better for those resulting from “pancreatic insufficiency.” It should be noted that the now popular glucagon-like peptide-1 (GLP-1) agonist, like tirzepatide (Monjaro) and semaglutide (Ozempic), are not yet approved for pregnant women and therefore not currently offered to subfertile women wanting to get pregnant at MFS. This may change in the future, given the result of a multicenter prospective study that compared women who were unintentionally exposed to this medication during the first trimester of their pregnancy with those who were not. This study found that the medication is not associated with the risk of major birth defects, with no difference in miscarriage and livebirth rate. (Dao K et al., BMJ Open. 2024;14(4):e083550). When oral medication is ineffective, women with type 2 diabetes and all who have type 1 diabetes will need insulin to control their blood sugar. We recommend strict control of diabetes before pregnancy to decrease associated pregnancy complications, preferring such women’s HbA1c to be 6.5% or lower.
To learn more about PCOS, please visit our Educational Resources Page.
Uterine Fibroids
Uterine Fibroids are slow-growing tumors of the uterine wall, but they are rarely found outside the uterus. They are not cancerous but can undergo malignant change in <1% of patients.Uterine fibroid does not necessarily cause subfertility but may sometimes do so if very large and if impinging on the uterine cavity or compressing the tubes.
Awonuga AO, Camp OG, Biernat MM, Abu-Soud HM. Overview of infertility. Syst Biol Reprod Med. 2025 Dec;71(1):116-142. doi: 10.1080/19396368.2025.2469582. Epub 2025 Mar 21. PMID: 40117219.
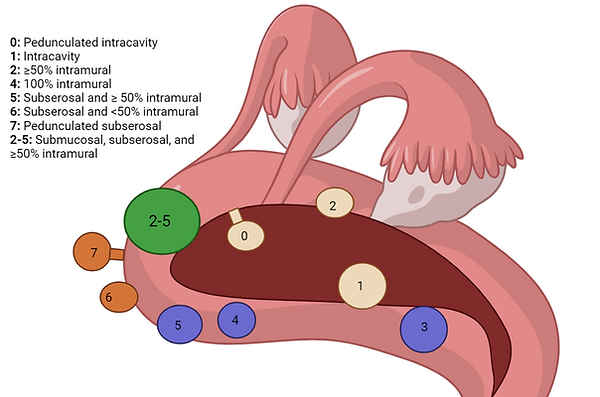
Uterine fibroids are classified by location according to their location relative to the uterine muscle. Most gynecologists, reproductive endocrinologists, and infertility specialists use the FIGO (International Federation of Gynecology and Obstetrics) classification system.
The main types are submucosal (projecting into the uterine cavity), intramural (within the myometrium), and subserosa (projecting outside the uterus).
These are further categorized based on their specific location within those regions as follows:
1. Submucosal fibroids are classified as
a. Type 0 (pedunculated and entirely within the uterine cavity)
b. Type 1 (< 50% intramural, i.e., within the uterine wall)
c. Type 2 (≥ 50% intramural, i.e., within the uterine wall).
d. Type 2-5 (< 50% within the uterine cavity relative to the endometrial cavity and
outside surface of the uterus termed the serosa)
2. Intramural fibroid with fibroid located entirely within the uterine wall.
a. Type 3 (100% within the muscular wall of the uterus and is also in contact with the
endometrium (the inner lining of the uterus).
b. Type 4 (like type 3, is 100% intramural fibroid, but unlike type 3 the fibroid does not
touch the endometrium and like type 3 does not touch the outer coat of the uterus).
3. Subserosal fibroids are classified as
a. Type 5 (≥ 50% intramural, i.e., within the uterine wall),
b. Type 6 (< 50% intramural, i.e., within the uterine wall)
c. Type 7 (pedunculated, i.e., attached to the outside surface of the uterus by a stalk).
4. Ectopic fibroids
a. Type 8 are found outside the uterus, such as the cervix or the broad ligament.
Women with uterine fibroids may present with no symptoms, their fibroids being detected for the first time following bimanual pelvic examination during annual pelvic examination or performance of pelvic ultrasound for reasons unrelated to fibroids.
When symptomatic, women with uterine fibroids may present with symptoms that include:
1. Metrorrhagia (irregular periods), menorrhagia (heavy or prolonged but regular menstrual bleeding), menometrorrhagia (heavy or prolonged and irregular periods)
2. Abdominal pressure or fullness
3. Pelvic pain
4. Dyspareunia (pain during intercourse).
5. Other symptoms may include fatigue and exhaustion caused by anemia from associated menorrhagia. Some may also experience urinary symptoms, constipation, or lower back pain.
Diagnosis of Uterine Fibroids
Uterine fibroids are usually multiple and vary in size, number, and location. They can be assessed by bimanual pelvic examination, but better by pelvic ultrasound performed in the early part of the menstrual cycle, usually on days 2-5, and by saline infusion sonohysterogram (SIS) performed after the menstrual period has stopped and before likely ovulation (usually days 6 to 12). The later days are chosen to reduce the likelihood of pushing the embryo in transit in the tube, back into the pelvic cavity. Some patients have adenomyosis co-existing with uterine fibroids. Some also have adenomyomas (when the presence of adenomyosis coalesces to form a mass). Adenomyomas have similarities with uterine fibroids on ultrasound, but have their peculiar characteristics. They can be easily differentiated by experience in performing pelvic ultrasounds, which Dr. Awonuga has been doing for over 20 years. Both ultrasound (US) and magnetic resonance imaging (MRI) are used to evaluate a uterus with many fibroids. While the US is often the first-line imaging tool due to its accessibility and lower cost, MRI can be more sensitive in detecting and characterizing fibroids, especially in cases of multiple or larger fibroids. Pelvis MRI may also be necessary when detailed information is required because it provides the most detailed information regarding the number, size, and exact location of all fibroids in the uterus, thereby providing the greater accuracy needed to plan treatment. MRI is also better than pelvic ultrasound in detecting associated pelvic adhesions and can distinguish an adenomyoma (a benign nodular growth composed of endometrial glands and stroma located within the uterine wall) from a uterine fibroid.
Treatment of Uterine Fibroids
Uterine fibroids are managed on the basis of the signs and symptoms specific to the patient. Surgical management of asymptomatic fibroids remains controversial. At MFS, we individualize management. Medical Management 1. Vitamin D is an antioxidant and has been shown to inhibit fibroid proliferation. Vitamin D does this by modulating growth factors (naturally occurring protein-like substances that stimulate growth of cells, proliferation, and differentiation) responsible for fibroid growth. It should be noted that the role of vitamin D in reducing the size of uterine fibroids has been reproduced in the Eker mouse model, where supplementation with 1,25 dihydroxyvitamin D3 at 0.5 μg/kg per day for 3 weeks, led to reduced myoma size compared to controls. 2. For patients with significant fibroids that could impact fertility, medical option is often not applicable. This is because it can take several months for the fibroid to shrink enough to allow infertility treatment. However, this may be applicable to a young woman in her early twenties who is not desperate to conceive right away. Options available to such a patient include the use of Elagolix, estradiol, and norethindrone acetate combination for the management of heavy menstrual bleeding associated with uterine leiomyomas. 3. When surgery becomes necessary, the objective is to preserve the integrity of uterine musculature (wall) while removing or destroying the fibroid. The surgical technique depends on the size, number and location of the fibroid relative to the uterine cavity and whether the latter is distorted; the skill of the surgeon; and availability of surgical instruments. Applicable techniques include: a. High-intensity Focused Ultrasound Ablation (non-invasive): A technique that uses high intensity focused ultrasound energy to precisely target, heat and destroy fibroid tissue, potentially reducing the need for surgery. b. Uterine Artery Embolization (UAE): A minimally invasive procedure that blocks the artery that supplies blood supply to the uterus. This can help shrink fibroids by blocking their blood supply. c. Surgical excision of uterine fibroids. Although can be done by laparotomy (opening the abdomen the old fashion way) is now done by laparoscopic excision, lately via the Robot. In both situation, techniques like the triple-flap method are being developed to minimize the risk of uterine rupture in future pregnancies.
Endometriosis and Adenomyosis
Adenomyosis is a gynecological condition in which endometrial tissue (the lining of the uterus) is found in the muscular wall of the uterus (myometrium). Because endometrial tissues are not supposed to be in the uterine wall, they are regarded as ectopic tissue in this location, and they cause thickening and enlargement of the uterus. Although not well known, this disease is similar to the well-known condition called endometriosis, in which endometrial-type tissue is found outside the uterus in the abdominal and pelvic cavity. About 80% of women with adenomyosis have endometriosis. Similarly, about 91% of women with endometriosis have adenomyosis.
Women with adenomyosis or endometriosis can present with pelvic and or lower abdominal pain, heavy (menorrhagia) and or prolonged bleeding period, pain at intercourse (dyspareunia), and fatigue, all of which can affect quality of life. Because endometriosis can be present on the bowel and bladder, women with this disease can also present with pain with bowel movements, diarrhea, constipation, or bloating, or during urination.
The associated menorrhagia can lead to anemia, while chronic pelvic pain and disrupted sleep patterns can contribute to feelings of fatigue and exhaustion. How bad each of these symptoms is can vary from person to person, and some individuals can have all these symptoms.
Inability to conceive is well documented in patients with endometriosis, often from distorted pelvic anatomy affecting the uterine tubes. The existing literature on the effect of adenomyosis on fertility is inconclusive; however, once pregnancy is achieved, there is evidence to suggest a slightly increased risk of miscarriage, cervical incompetence, gestational hypertension, preeclampsia, fetal growth restriction, and preterm labor. These adverse pregnancy outcomes are more likely if the uterus is enlarged and the adenomyosis is diffuse.
Treatments
Treatment options depend on the severity of symptoms. When symptoms are mild, patients can often proceed to their chosen treatment after completing infertility investigations. At MFS, we frequently advise that an anti-inflammatory diet may help reduce any associated inflammation, potentially slowing the progression of the disease. This diet includes consuming foods such as fruits, vegetables, whole grains, and healthy fats, while limiting inflammatory triggers like processed foods, refined carbohydrates, and unhealthy fats.
When treatment is necessary, an infertility treatment regimen could be chosen that would manage symptoms and improve fertility. For example, when ovarian stimulation is required, either intrauterine insemination (IUI) or IVF could be done using:
1. Medications shown to help control heavy bleeding and dysmenorrhea before starting ovulation induction:
a. Tranexamic Acid (TXA)
b. Mifepristone
c. Valproic acid
2. Patients can be put on combined oral contraceptives, vaginal rings or progestin-only pills to help regulate menstrual cycles and reduce bleeding, for some time
before commencing ovulation induction.
3. An aromatase inhibitor like Letrozole reduces testosterone's conversion to estrogen, thus suppressing enhancement of lesions and inflammation.
a. Aromatase inhibitors are cheaper than gonadotropin-releasing hormone agonists (GnRH-a), which produce a more sustained estrogen suppression that can
help reduce symptoms and inflammation, potentially improving the uterine environment for implantation. This regimen is more commonly used when IVF
becomes necessary.
Rarely, conservative surgical management may be necessary. These surgical techniques preserve the uterine wall and surrounding tissue while removing or destroying the endometriotic or adenomyotic tissue.
Our Story
Since 1992, Dr. Awonuga has been helping couples and women achieve their dream of taking a baby home following infertility treatment. Once practiced as Division and Fellowship Program Director at Wayne State University/Wayne Health Reproductive Endocrinology and Infertility practice, he has now opened Michigan Fertility Services. Dr. Awonuga is an active researcher and continues to collaborate with scientists at the C.S. Mott Center for Human Growth and Development at Wayne State University. With extensive experience in the field, at Michigan Fertility Services we will educate our patients and be gentle, ethical, and respectful. These are essential because as inability to conceive and have children is associated with anxiety and psychological stress. Dr. Awonuga understands and will help manage these facets with an appropriate referral if necessary.
